Cefdinir Impurity V |
|
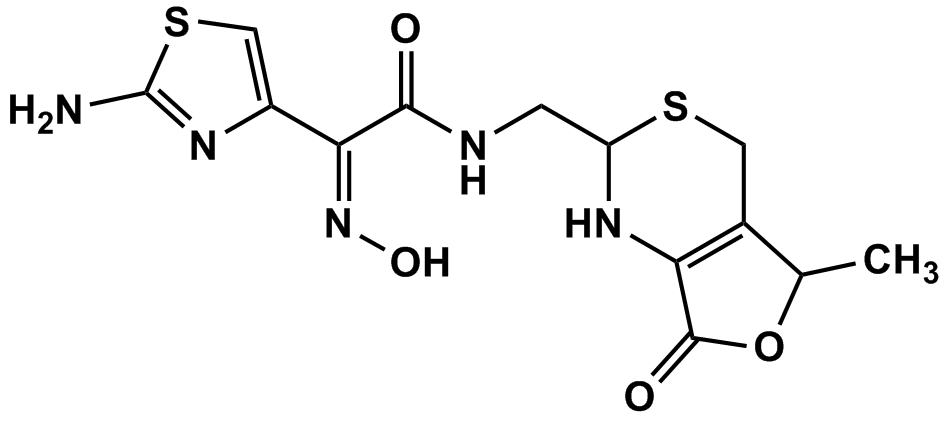
| Synonyms: [2RS-[2a(Z),5a]]-2-Amino-a-(hydroxyimino)-N-[(1,2,5,7-tetrahydro-5-methyl-7-oxo-4H-furo[3,4-d][1,3]thiazin-2-yl)methyl]-4-thiazoleacetamide; (Z)-2-(2-Aminothiazol-4-yl)-2-(hydroxyimino)-N-{[(2RS,5RS)-5-methyl-7-oxo-2,4,5,7-tetrahydro-1H-furo[3,4-d][1,3]thiazin-2-yl]methyl}acetamide;Cefdinir Impurity 3 (Mixture of 2 Diastereomers) | |
| Cat. No.: C21125 | CAS No.: 178949-04-7 |
| MDL.: | Formula: C13H15N5O4S2 |
| F.W.369.42 | Purity: 99% |
| Storage: No Data Available | |
| Catalog Number: | C21125 | CAS No.: | 178949-04-7 | |
| MDL: | Formula: | C13H15N5O4S2 | ||
| F.W.: | 369.42 | Purity: | 99% | |
| Package: | Unit: | mg | ||
| Appearance: | No Data Available | M.P.: | No Data Available | |
| B.P.: | Density: | |||
| Optical Rotation: | Refractive Index: | |||
| Solubility: | No Data Available | Stability: | ||
| Storage: | No Data Available | Category: | Reference Standard | |
| Reference: | Inamoto, Y., et al.: J. Antibiot., 41, 828 (1988), Ternansky, R., et al.: J. Antibiot., 46, 189(1993), | |||
| Application: | Cefdinir impurity formed by hydrolytic degradation pathway | |||
| Category: | Chiral Reagents, Impurity, Pharmaceuticals, Intermediates & Fine Chemicals, Sulfur & Selenium Compounds, | |||








