Cefdinir Impurity M |
|
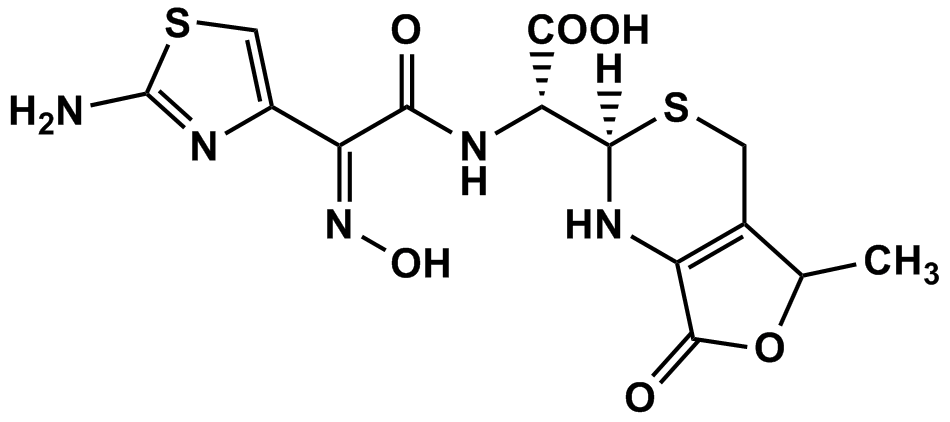
| Synonyms: [2RS-[2a[RS*(Z)],5ß]]-a-[[(2-Amino-4-thiazolyl)(hydroxyimino)acetyl]amino]-1,2,5,7-tetrahydro-5-methyl-7-oxo-4H-furo[3,4-d][1,3]thiazine-2-acetic Acid; 2(R)-2-[(Z)-2-(Aminothiazol-4-yl)-2-(hydroxyimino)acetamido)]-2-[(2RS,5RS)-5-methyl-7-oxo-2,4,5,7-tetrahydro-1H-furo[3,4-d][1,3]thiazin-2-yl]acetic Acid;Cefdinir Impurity 4 (Mixture of 4 Diastereomers) | |
| Cat. No.: C21116 | CAS No.: 178422-45-2 |
| MDL.: | Formula: C14H15N5O6S2 |
| F.W.413.43 | Purity: 99% |
| Storage: No Data Available | |
| Catalog Number: | C21116 | CAS No.: | 178422-45-2 | |
| MDL: | Formula: | C14H15N5O6S2 | ||
| F.W.: | 413.43 | Purity: | 99% | |
| Package: | Unit: | mg | ||
| Appearance: | No Data Available | M.P.: | No Data Available | |
| B.P.: | Density: | |||
| Optical Rotation: | Refractive Index: | |||
| Solubility: | No Data Available | Stability: | ||
| Storage: | No Data Available | Category: | Reference Standard | |
| Reference: | Inamoto, Y., et al.: J. Antibiot., 41, 828 (1988), Ternansky, R., et al.: J. Antibiot., 46, 189(1993), | |||
| Application: | Cefdinir impurity formed by hydrolytic degradation pathway | |||
| Category: | Chiral Reagents, Impurity, Pharmaceuticals, Intermediates & Fine Chemicals, Sulfur & Selenium Compounds, | |||








