Cefadroxil Impurity B |
|
| Synonyms: (6R,7R)-7-amino-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; 7-aminodesacetoxycephalosporanic acid; 7-ADCA;EP Cefalexin Impuirty B;USP Cefadroxil Related Compound B; EP Cefadroxil Impurity B;EP Cephradine Impurity A | |
| Cat. No.: C20202 | CAS No.: ?22252-43-3? |
| MDL.: | Formula: C8H10N2O3S |
| F.W.214.24 | Purity: 99% |
| Storage: -20°C Freezer | |
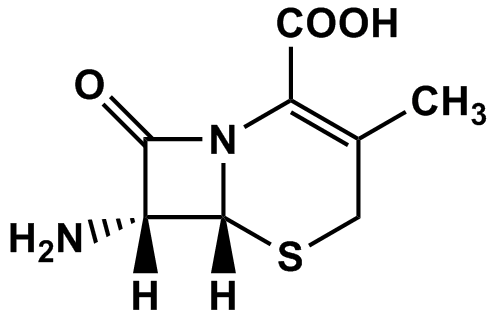
| Catalog Number: | C20202 | CAS No.: | ?22252-43-3? | |
| MDL: | Formula: | C8H10N2O3S | ||
| F.W.: | 214.24 | Purity: | 99% | |
| Package: | Unit: | mg | ||
| Appearance: | Off-White Solid | M.P.: | >225°C (dec.) | |
| B.P.: | Density: | |||
| Optical Rotation: | Refractive Index: | |||
| Solubility: | DMSO (Sparingly) | Stability: | ||
| Storage: | -20°C Freezer | Category: | Reference Standard | |
| Reference: | Dantzig, A., et al.: Biochim. Biophys. Acta, 1027, 211 (1990), Shen, H., et al.: J. Biol. Chem., 278, 4786 (2003), Watanabe, C., et al.: Drug Metab. Pharmacokinet., 20, 443 (2005) | |||
| Application: | A metabolite of Cephalexin; and an impruity of Cefradine | |||
| Category: | Metabolites & Impurities, Pharmaceuticals, Intermediates & Fine Chemicals, | |||








